Case Report
Distal Pancreatectomy with SmartPAN®
Authors: Dr. Pausch, Thomas; El-Mahdy, Josefin. University Hospital Heidelberg, Germany
Patient
- Female, 30 years, BMI: 21,7, Pancreas-type B (not-soft texture,
small duct) - Patient was in good general condition (ASA I), no comorbidities
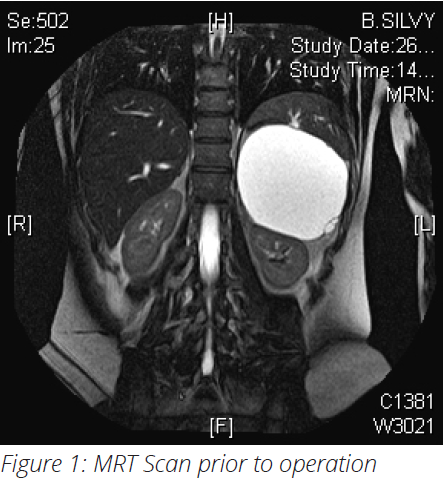
- MRI-Scan showed pancreatic lesion (Fig 1)
- Diagnosis: benign multicystic neoplasm located at the
pancreatic tail, suspected malignant potential - Patient was planned for exploration laparotomy and distal
pancreatectomy
SmartPAN®
- First-in-class medical device to indicate pancreatic leakage during surgery.
- Instructions:
- Rinse and dab operation field to ensure cleanliness and blood dryness
- Application of 4ml SmartPAN® on pancreatic remnant.
- Wait for 3 minutes and document color change.
- Rinse and suction to avoid accumulation in the body cavity.
- Indicator changes color from orange to blue upon contact with pancreatic fluid specifically and with high
optical resolution. - Direct visualization of pancreatic leakage in the surgical field.
Therapy: Distal pancreatectomy & SmartPAN® usage
- Patient received an open distal pancreatectomy with Splenectomy in general anesthesia
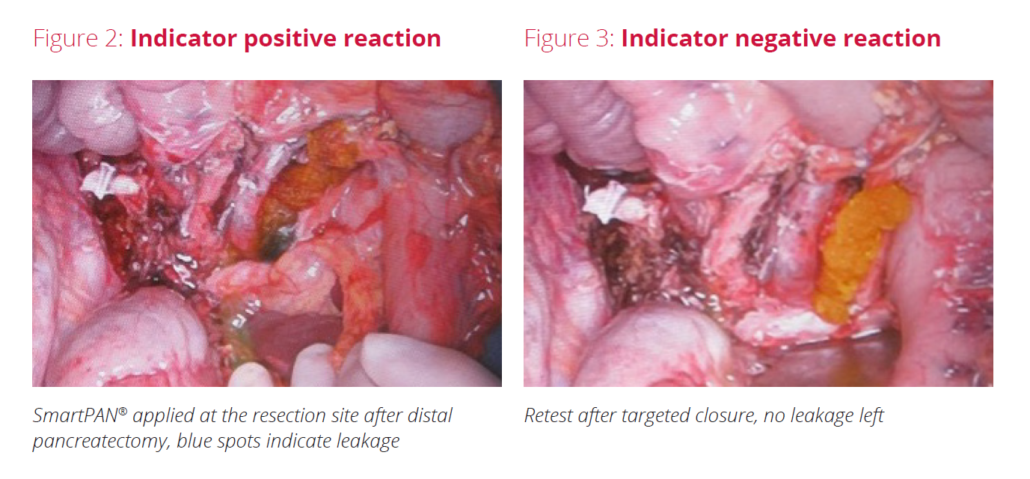
- Application of SmartPAN® at the resection site: indicator positive reaction showed leakage (Fig 1a)
- Surgeon applied 2 stitches for targeted closure
- Reapplication of SmartPAN® after targeted closure ➞ No color change visible, closure attempt successful
Outcome
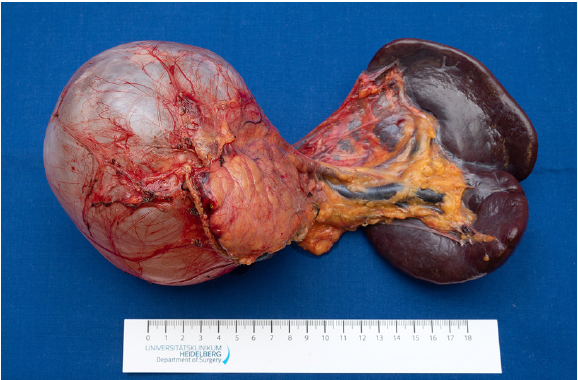
- Diameter of removed lesion: 12 cm (Fig. 4)
- Histology: pancreatic mucinous cystic neoplasm (MCN)
with low grade epithelial dysplasia, R0 resection - Patient showed no complications during the 30 day
follow-up after the operation - There were no signs of clinically relevant postoperative
pancreatic fistula in the drainage fluid and the clinical
course. Postoperative day 2: serum: Amylase 31 U/l,
Lipase 45 U/l ; drainage: Amylase 196 U/l, Lipase 412
U/L; drainage concentration on postoperative day 5:
Amylase 16 U/l, Lipase 35 U/l - Drainage could be removed after 6 days
- Patient was discharged from the hospital 7 days after distal pancreatectomy
- Toxicological analysis of central venous blood and drainage fluid, collected during the operation and on
postoperative day 2, showed no traces of the only non-biocompatible indicator component bromothymol
blue (BTB)
CONCLUSION
- SmartPAN® is easy to use during the operation
- The surgeon can perform targeted closure of the colored spots indicating leakage to
prevent postoperative complications following POPF - The toxicological analysis confirmed the safety and showed no accumulation of BTB
in the central venous blood stream or drainage fluid of the patient