Case report 2
KEEPING CRC LIVER METASTASES UNDER CONTROL WITH
DSM-TACE: A LONG TERM FOLLOW UP REPORT

Author: Simone Ciaglia, MD and Pierleone Lucatelli, MD, PhD
Interventional Radiologists
Policlinico Umberto I; Sapienza University Rome, Italy
Patient
- 62-year-old male
- In 2015, a CT scan revealed sigmoid colon cancer with multiple liver metastases located in both
hepatic lobes - 11/2015: laparotomy with sigmoid resection and adjuvant systemic chemotherapy
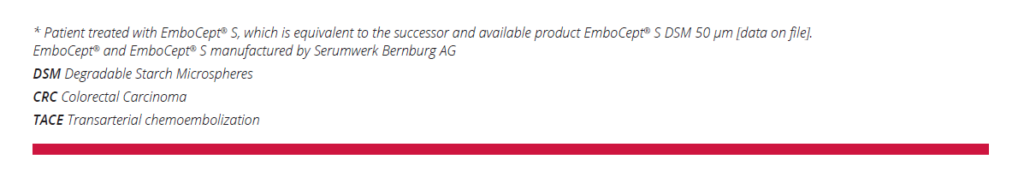
- 02/2016: CT scan performed after chemotherapy showed a reduction in size of the hypovascular multifocal liver
metastases located in the hepatic segments VIII, VI, IV, and I, Fig.1 - 04/2016 Tumor board decision: DSM-TACE with 450 mg EmboCept® S and 100 mg Irinotecan to treat liver metastases

DSM-TACE Procedure
- Four sessions of bilobar DSM-TACE were completed within one year
- Procedures were performed in angiography suite under local anesthesia
- Right femoral approach, 4 F diagnostic catheter. The patient had a variant anatomy of the visceral arteries with the left hepatic artery originating from the left gastric artery. The diagnostic catheter was first positioned in the proper hepatic artery, and a 2.4-F microcatheter was placed in the right hepatic artery to perform selective embolization of the right lobe. Subsequently, the diagnostic catheter was positioned in the left gastric artery, and the microcatheter was advanced into the left hepatic artery to perform selective embolization of the left lobe.
- Under radiographic guidance, a solution of 450 mg EmboCept® S suspended in 7.5 ml injection solution, 100 mg Irinotecan, and contrast medium were slowly infused in a stepwise manner:
- 1. 210 mg EmboCept® S (~ 3,5 ml) were mixed with 100 mg Irinotecan and contrast agent (350 mg/ml), resulting in a total volume of 10 ml: solution “A”.
- 2. 240 mg EmboCept® S were mixed with contrast agent (350 mg/ml), resulting in a total volume of 10 ml: solution “B”.
- Step 1: Solution A was administered by manual injection.
- Step 2: Solution B was added as a plug by manual injection, until anterograde flow reduction was obtained.
- The endpoint for a successful treatment was defined as the delivery of the full planned dose and reduction of anterograde artery flow.
- After successful administration, the 5 F sheath was removed, and a vascular closure device was applied for hemostasis.
Outcome
- No serious adverse events occurred in the patient, and only a transient ALT/AST elevation was observed.
- The patient experienced fatigue for about one week without other signs of post-embolization syndrome and without any impairment of the personal activity level.
- Control CT scan one month after the fourth chemoembolization revealed an excellent response, the metastases
were almost undetectable Fig. 2.

- Patient underwent to systemic chemotherapy for the next three years and stable disease was observed.
- A CT scan performed on 06/2020 revealed an increase in size of the lesion located in the segments IV and VI
and a new lesion in the segment V was observed. - According to liver tumor board decision, patient underwent resection of the segment IV and another TACE
treatment was proposed for the other lesions. - Within six months, the patient underwent another three DSM-TACE (EmboCept® S DSM 50 μm + Irinotecan) treatments.
- The MRI control performed after the three DSM-TACE treatments on 10/2021, revealed stable disease of the lesions
located on the segments V, after VI and VIII, but an increase of the lesion located in left hepatic lobe (segment II). - Two more DSM-TACE treatments (EmboCept® S DSM 50 μm + Irinotecan) in the left hepatic lobe were proposed
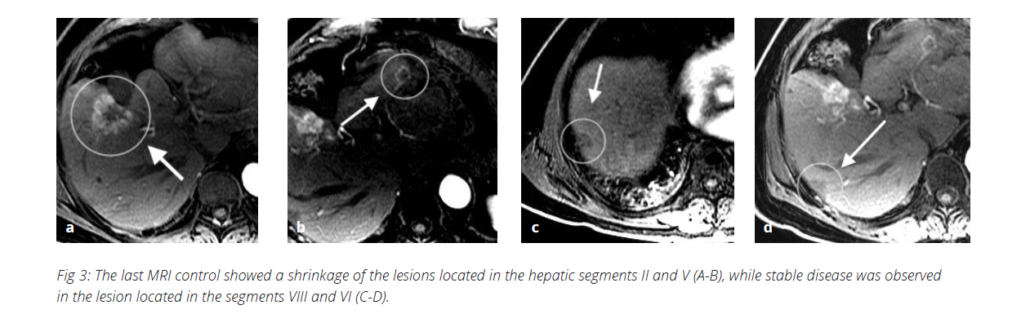
and performed within three months. - The last MRI control on 03/2022 showed a shrinkage of liver metastases located in the segments V and II and
stable disease of the lesion located in segments VIII and VI Fig. 3.
CONCLUSION
- EmboCept® S DSM 50 μm is an effective, safe and easy to use embolic agent.
- As EmboCept® S DSM 50 μm is degradable, DSM-TACE can be used to treat the whole organ thanks to its low toxicity and permits repeated use of the same vessels.
- EmboCept® S DSM 50 μm can be associated with any chemotherapy suited for transarterial administration.
- DSM-TACE can be successfully used to treat colorectal liver metastases, permitting multiple treatments thanks to its low toxicity and good tolerability.